IGNOU Assignments Solutions | Ignou Question Paper & Updates
EVERYTHING - IS FREE FOR EVERY STUDENT!!!
EVERYTHING - IS FREE FOR EVERY STUDENT!!!
Bachelor’s Degree Programme (BSCG) – CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I Solved Assignment Answer | BCHCT 133 | 2021-2022

Free BCHCT 133 Solved Assignment Answer | CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I | For July 2021 – June 2022
IGNOU BCHCT 133 Solved Assignment, This assignment is the curriculum of the BACHELOR’S OF SCIENCE (BSCG) program. For other courses, students check our website categories section to find their courses and assignments over there.
BCHCT 133 CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I Solved Assignment 2021-2022
IGNOU BCHCT-133 CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I assignment are 100 marks. There are two sections – A and B. Students have to answer all the questions. Download IGNOU BCHCT-133 Assignment free without any registration, Students can easily download the IGNOU assignment question paper from the official website of the university. They are not required to pay any fees or charges for this.
| Title Name | BCHCT-133 Solved Assignment 2021-2022 | CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I |
| University | IGNOU |
| Service Type | Solved Assignment (Soft Copy/Q&A form) |
| Course | BSCG |
| Language | English |
| Semester | Session: July 2021 – January 2022 |
| Short Name | BCHCT-133 |
| Assignment Code | BCHCT-133/TMA/January 2022 |
| Product | Assignment of BCHCT-133 | 2021-2022 |
| Submission Date | Valid from 1st July 2021 to 30th June 2022 |
| Assignment Pdf | Download Now |
How to get CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I, BCHCT 133 Solved Assignment?
It is always good to solve the assignment by yourself. Because it helps the students in the whole study. It also helps them in preparing for the exam. So that the question that comes in the exam can be solved easily.
If someone is unable to solve the assignment, so don’t worry we have solved this assignment for you, you only need to click the link one by one and get all the answers to this assignment sequence-wise. And if not, then our team is working on it and you will be available soon.
The answer of BCHCT-133 | CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I | Solved Assignment 2021-2022:
Note: Attempt all questions. The marks for each question are indicated against it.
Part A: CHEMICAL ENERGETICS AND EQUILIBRIA
1. a) Define chemical thermodynamics and outline its significance.
b) Explain different types of thermodynamic systems with suitable examples.
c) Derive an expression for isothermal reversible expansion of a gas from a volume V1 to V2.
2. a) Describe the method for experimental determination of energy changes accompanying chemical reactions under constant volume conditions.
b) Explain the significance of rU and rH and derive the relationship between them.
c) Predict the enthalpy of hydrogenation of 1-propene using the bond enthalpy data from Table 3.2 of (Unit 3; p, 76).
3. a) Define the spontaneity of a reaction and give the criteria of the spontaneity of reactions in terms of i) entropy and ii) Gibbs energy.
b) Derive an expression for the entropy change for isothermal Mixing of Ideal Gases.
c) Define equilibrium constant and for the following reaction:
2NH3(g) ⇌ 2N2(g) +3H2 (g)
i. Write the expression for Kc.
ii. Relate Kc to Kp, and
iii. Relate Kc to Kx
4. a) Define the degree of ionization of a weak electrolyte and list the factors affecting it.
b) What are amphoteric substances? Explain with the help of suitable examples.
c) State Le- Chatelier’s principle and for the following reaction at equilibrium:
2SO2(g) + O2(g) ⇌ 2SO3(g) ∆H = 198 kJ
Predict the effect of
i. Adding SO2(g) at equilibrium
ii. Decreasing the temperature
iii. Reducing the volume of the container to half
iv. Adding an inert gas under conditions of constant pressure.
5. a) Define solubility and give the relationship between solubility and solubility product constant for sparingly soluble salts of AB types.
b) Calculate the degree of ionisation and the pH of a 0.01 M aqueous solution of formic acid at 298K. [Given, Ka (HCOOH) = 1.7 10−4at 298K].
c) Calculate the pH of 0.01 M aqueous solution of sodium formate at 298 K. [Given: Ka (HCOOH) =1.7 × 10−4 at 298K]
Part B: FUNCTIONAL GROUP ORGANIC CHEMISTRY-I
6. Write chemical equation(s) for the following conversions :
i) Phenol to benzene
ii) Ethylbenzene to 1-chloro-1-phenylethane
iii) Aniline to chlorobenzene
iv) Phenol to 4-hydroxybenzaldehyde
v) Anisole to phenol
b) Write the mechanism of the Friedel-Crafts alkylation reactions.
7. a) What do you understand by ortho and para-directing activator? Explain with the help of a suitable example.
b) Write the mechanism of the reaction of allyl chloride with water.
8. Explain the following:
a) Reaction of silver cyanide with bromopropane gives 1-propylisonitile not npropyl cyanide.
b) Ether is the major product in the reaction of tert-butylbromide with ethanol
9. a) Why thionyl chloride is generally used to convert alcohols to alkyl halides? Taking suitable example write the mechanism of the reaction of an alcohol with thionyl chloride.
b) How you will distinguish between aldehydes and ketones?
10. a) Write the mechanism for Knoevenagel condensation.
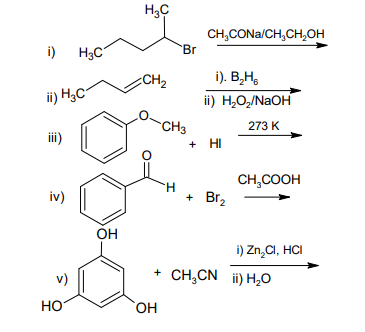
b) Complete the following reactions:
Wrapped Up
- For More BSCG Assignments – BSCG Solved Assignment
- For Hand Written Assignments – Click here
For more Updates join our Telegram Group and also for your inquiry you may comment here or mail us –info@ignouassignmentssolutions.in
Telegram Group – Click Here
- bchct 133 solved assignment
- ignou assignment 2020
- ignou assignment 2021
- ignou assignments
- ignou solved assignment
- ignouassignmentstatus
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 25 | 26 | 27 | 28 | 29 | 30 | |
Recent Posts
- IGNOU Hall Ticket June 2023 – Check TEE June 2023 Admit Card Ignou
- IGNOU BHIC 104 Previous Year Question Paper & Important Question | Arts (Honours) Ignou Question Paper
- IGNOU BHIC 103 Previous Year Question Paper & Important Question | Arts (Honours) Ignou Question Paper
- IGNOU BHIC 102 Previous Year Question Paper & Important Question | Arts (Honours) Ignou Question Paper
- IGNOU BHIC 101 Previous Year Question Paper & Important Question | Arts (Honours) Ignou Question Paper
Recent Comments
Top Categories
Sign Up to Our Newsletter!!!

Bachelor’s Degree Programme (BSCG) - MOLECULES OF LIFE Solved Assignment Answer | BCHET 149 | 2021-2022

Bachelor of Art General & Honours (BAG) - INTRODUCTION TO SOUTH ASIA Solved Assignment Answer | BPSE 144 | 2021-2022

Bachelor’s Degree Programme (BSCG) - CHEMICAL ENERGETICS, EQUILIBRIA AND FUNCTIONAL ORGANIC CHEMISTRY-I Solved Assignment Answer | BCHCT 133 | 2021-2022

Bachelor of Art General & Honours (BAG) - Business Communication Solved Assignment Answer | BEGS 186 | 2022-2023

What do you understand by plantation to which the plantation Labour Act would be applicable? What are the provisions relating to health in the plantation?

IGNOU BHIC 104 Previous Year Question Paper & Important Question | Arts (Honours) Ignou Question Paper

IGNOU BCOC 138 Previous Year Question Paper & Important Question | I.A.S.

Bachelor of Science Honours in Biochemistry (BSCBCH) - HUMAN PHYSIOLOGY Solved Assignment Answer | BBCCT-115 | 2021-2022

IGNOU BCHET 141 Previous Year Question Paper & Important Question | Chemistry Ignou Question Paper

IGNOU BECC 102 Previous Year Question Paper & Important Question | I.A.S.

Bachelor of Art General & Honours (BAG) - English Language Teaching Solved Assignment Answer | BEGS 185 | 2022-2023

Bachelor of Art General & Honours (BAG) - Business Communication Solved Assignment Answer | BEGS 186 | 2022-2023

Bachelor of Art General & Honours (BAG) - English Communication Skills Solved Assignment Answer | BEGAE 182 | 2022-2023

Master's Degree Programme (MSW-C) - Fields of Counselling Solved Assignment Answer | MSW-016 | 2021-2022

IGNOU ECO 06 Previous Year Question Paper & Important Question | Commerce Ignou Question Paper

IGNOU ECO 05 Previous Year Question Paper & Important Question | Commerce Ignou Question Paper

IGNOU ECO 03 Previous Year Question Paper & Important Question | Commerce Ignou Question Paper






